In Hypertonic Solutions Water Moves Blank the Cell
Cytosol and ECF have approximately the same concentration of solute. Since water tends to flow out of the cell cells placed in a hypertonic solution will shrink.
This causes the cell to shrivel.
. Water potential by definition is the potential developed due to the concentration of free water molecules in a system or solution. A high amount of water loss can be. Water will move from low to high.
Start studying Cells and Tonicity. Osmotic pressure pulls water into the cell. In a hypertonic solution of NaCl there is _____ NaCl outside the red blood cell so there will be _____ free water molecules outside the cell than inside it.
Tonicity is actually a phrase which explains the mode of concentration of a certain solution in terms of hypertonicity hypotonicity or isotonicity. This will decrease the concentration of water molecules inside the cell. When a solute has been dissolved in water there is a relative decrease in a number of solvent or water molecules.
Cells that lose too much water. Therefore the water moves _____ the cell. Aboth into and out of the cell.
In this case also there is a decrease in water potential of the cell. Start studying Biology FLIP BOOK. Advertisement Advertisement 24emmaleerowell 24emmaleerowell Answer.
Water will move from low to high. In an isotonic environment there is no net water movement so there is no change in the size of the cell. When a plant cell is placed in a hypertonic solution what occurs.
Hypertonic isotonic hypotonic solutions. Hence water potential decreases. In a hypertonic solution of NaCl there is _____ NaCl.
Therefore there is no net movement of water. B because its a hypertonic solution. Movement of Water in Hypertonic Solutions Water moves across a semipermeable membrane.
Therefore the cell loses water and shrinks. Learn vocabulary terms and more with flashcards games and other study tools. In a hypertonic solution water will move ____ because there are too many solutes outside of the cell.
Nov 22 2016 In a hypotonic solution water moves into the cell by endosmosis. For example when we. Hence when water moves out the water potential of cell decreases.
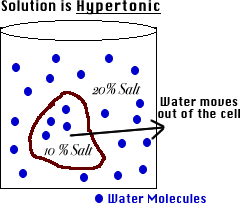
In this case the opposite will happen as water moves out of the cell. Water inside the cell highest concentration moves out of the cell lowest concentration causing the plant cell to shrink and the plant to wilt. We know that hypertonic solution is a solution in which the concentration of the solute is more outside the cell than inside the cell.
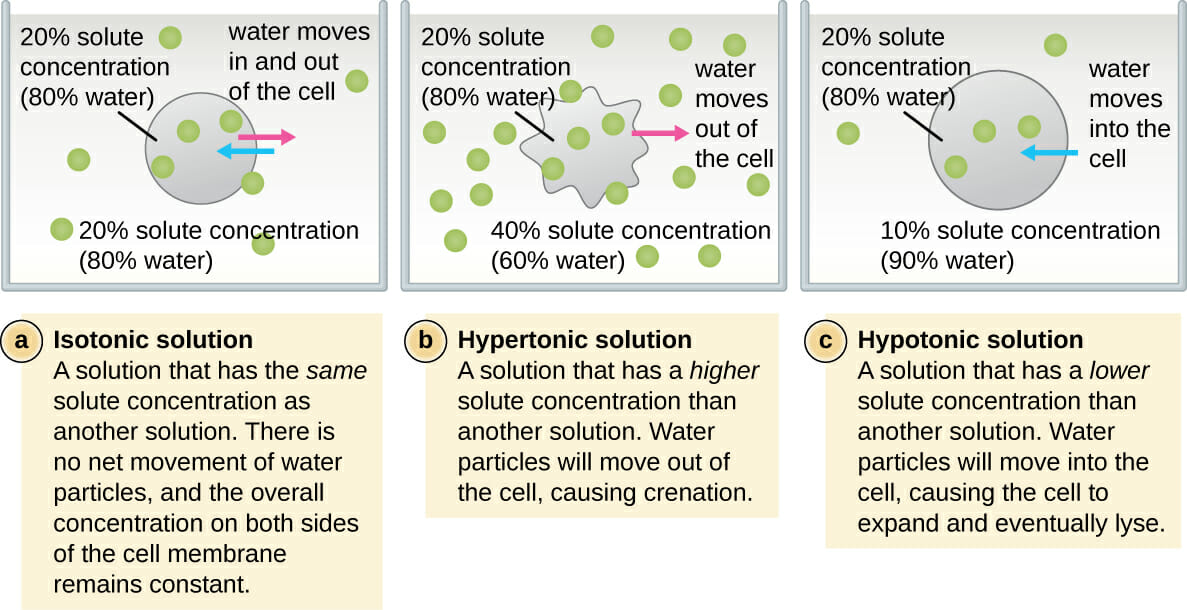
Remember water moves to equalize the concentration of solute particles. Solute concentration of the ECF is higher than inside the cell. Solute concentration of the ECF is lower than inside the cell.
If the solutions on either side of the membrane are. If enough water is lost the cell will take on a wrinkled or shriveled appearance. Too few solutes in the environment will become the hypertonic solution.
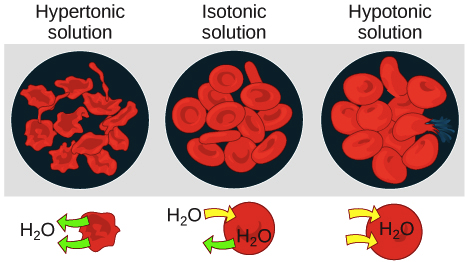
Hypertonic solution have higher osmotic pressure. If placed in a hypotonic solution a red blood cell will bloat up and may explode while in a hypertonic solution it will shrivelmaking the cytoplasm dense and its contents concentratedand may die. When we place a cell in a hypertonic solution then the water moves out of the cell and gradually moves towards the area of higher solute concentration.
Learn vocabulary terms and more with flashcards games and other study tools. If a cell is placed in a hypertonic solution water will leave the cell and the cell will shrink. In red blood cells this is called crenation and the surface of the cells take on a scalloped appearance.
When a cell is placed in a hypotonic environment water will. The process by which water moves out a cell in a hypertonic solution is called plasmolysis. If a cell is placed in a hypertonic solution then water will flow from inside the cell to outside.
Osmotic pressure pulls water out of the cell so the cell crenates shrinks Hypotonic. Again when we reference a solution to say it is hyper and hypo we are referencing the amount of solute present in the solution in. Upload your study docs or become a Course Hero member to access this document Continue to access End of preview.
A solution will be hypertonic to a cell. Therefore a hypertonic solution has more solutes than the intracellular environment so water will leave the cell to try to achieve equilibrium. Three termshyerptonic hypotonic and isotonicare used to describe whether a solution will cause water to move into or out of a cell.
Being that the outside solution is 90 water while the inside contains 991 water water flows from the inside of the cell to the outside solution to dilute the high areas of solute concentration. It would not survive because it would be placed in a hypertonic environment and so the water will move out of the cell forcing it to shrivel up and die. That means its concentrated so water moves out of the cell into the solution.
If a cell is placed in a hypertonic solution water will leave the cell and the cell will shrink. When a cell is placed in a hypertonic solution then water moves out of the cell due to the process of exosmosis. Thus in a hypertonic solution water leaves the cell and moves to the environment.
When a cell is placed in a hypertonic solution osmotic pressure will force water out of the cell to balance the concentration of solute across the membrane. Water moves against the concentration gradient of solutes moving from areas of low solute concentration to areas of high solute concentration. The plant wilts because there is a loss of turgor pressure.
If a cell is placed in a hypertonic solution there will be a net flow of water out of the cell and the cell will lose volume.
Isotonic Vs Hypotonic Vs Hypertonic Solution Biology
What Is The Movement Of Water Molecules From Hypertonic To Hypotonic Solution Quora
No comments for "In Hypertonic Solutions Water Moves Blank the Cell"
Post a Comment